- 电话:+1 2027805918 传真:+1 208640718 邮箱:info@seedoctorsinusa.com 国内电话:400-7800-120
- Copyright © 2014 - 2017 Z&L International Medical(Z&L) All Rights Reserved.
- SeeDoctorsInUSA 美国好大夫(美国好医生)operated by Z&L International Medical, LLC | Technical Support by Jing
FDA 批准了第一个用于I 型糖尿病的“人工胰腺”
FDA Approves First 'Artificial Pancreas' for Type 1 Diabetes
Miriam E Tucker|September 28, 2016
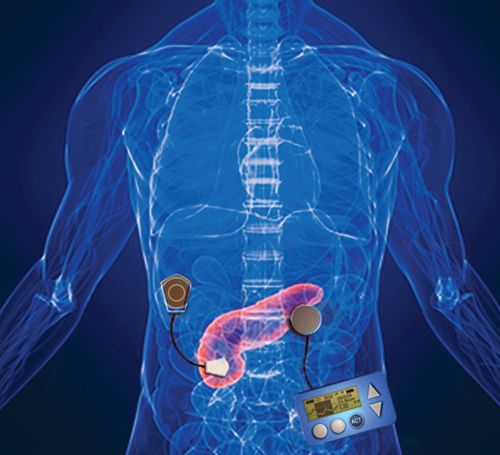
The US Food and Drug Administration (FDA) has approved Medtronic's MiniMed 670G hybrid closed-loop insulin delivery system, the first-ever device that automatically monitors blood glucose and administers appropriate basal insulin doses, for patients aged 14 years and older with type 1 diabetes.
Because the device responds to both low and high blood glucose levels — the current Medtronic system corrects only lows — it is being called the first-ever "artificial pancreas." However, since patients still need to enter information about anticipated meals and request the device to deliver bolus insulin doses, it is called a "hybrid" rather than a fully closed-loop system.
The system comprises a subcutaneously worn continuous glucose monitor that measures glucose levels every 5 minutes and an insulin pump that displays glucose readings and delivers insulin based on those values. Prior to meals, patients approximate the carbohydrate count of their food and enter that information in so that the system can calculate the needed bolus dose.
The FDA approval was based on pivotal trial data on 124 type 1 diabetes patients aged 14 to 75 years, showing that when they wore the 670G for 3 months, it produced reductions of both hypoglycemia and hyperglycemia with less glycemic variability compared with baseline data.
During the study, HbA1c levels dropped from 7.4% at baseline to 6.9%, and the percentage of sensor glucose values within the target range changed from 66.7% at baseline to 72.2%. No serious adverse events, diabetic ketoacidosis, or severe hypoglycemia were reported.
Those data were recently published in Journal of the American Medical Association and also presented by Richard M Bergenstal, MD, executive director of the International Diabetes Center, Minneapolis, Minnesota, at the European Association for the Study of Diabetes (EASD) 2016 Annual Meeting.
"After 30-plus years of promising patients that we would have an artificial pancreas, I was never quite certain I'd see it in my career. [Now] I think we're actually going to see it in the near future," Dr Bergenstal told Medscape Medical News at the EASD conference.
However, an investigator for a competing product, Steven J Russell, MD, of the Diabetes Research Center at Massachusetts General Hospital, Boston, expressed the view that although it does reduce glycemic variability, the 670G represents a "conservative strategy" because of the ongoing need for patients to estimate their carb consumption, which can introduce error in insulin dosing.
In a statement, the FDA noted that risks associated with use of the system may include hypoglycemia and hyperglycemia, as well as skin irritation or redness around the device's infusion patch. In addition, this version of this device is unsafe for use in children 6 years of age or younger and in patients who require less than eight units of insulin per day.
As part of this approval, the FDA is requiring a postmarket study to better understand how the device performs in real-world settings. Medtronic is currently performing clinical studies to evaluate the safety and effectiveness of the device in children 7 to 13 years old with type 1 diabetes.
Dr Bergenstal has conducted research, served on a scientific advisory board, or consulted for Medtronic, Dexcom, Roche, Abbott, Johnson & Johnson, Eli Lilly, Novo Nordisk, and Sanofi. He receives no personal income from these activities, as all contracts are with his nonprofit employer HealthPartners Institute. He has inherited stock in Merck. Dr Russell has received honoraria from Tandem and Novo Nordisk, lecture and other fees from Tandem, Sanofi, Dexcom, and Eli Lilly and nonfinancial support from Dexcom, Tandem Diabetes, SweetSpot Diabetes, International Biomedical, Abbott Diabetes Care, Insulet, and Medtronic. He owns stocks/shares in Companion Medical, and has patents pending on the bionic pancreas.
Miriam E Tucker|September 28, 2016

The US Food and Drug Administration (FDA) has approved Medtronic's MiniMed 670G hybrid closed-loop insulin delivery system, the first-ever device that automatically monitors blood glucose and administers appropriate basal insulin doses, for patients aged 14 years and older with type 1 diabetes.
Because the device responds to both low and high blood glucose levels — the current Medtronic system corrects only lows — it is being called the first-ever "artificial pancreas." However, since patients still need to enter information about anticipated meals and request the device to deliver bolus insulin doses, it is called a "hybrid" rather than a fully closed-loop system.
The system comprises a subcutaneously worn continuous glucose monitor that measures glucose levels every 5 minutes and an insulin pump that displays glucose readings and delivers insulin based on those values. Prior to meals, patients approximate the carbohydrate count of their food and enter that information in so that the system can calculate the needed bolus dose.
The FDA approval was based on pivotal trial data on 124 type 1 diabetes patients aged 14 to 75 years, showing that when they wore the 670G for 3 months, it produced reductions of both hypoglycemia and hyperglycemia with less glycemic variability compared with baseline data.
During the study, HbA1c levels dropped from 7.4% at baseline to 6.9%, and the percentage of sensor glucose values within the target range changed from 66.7% at baseline to 72.2%. No serious adverse events, diabetic ketoacidosis, or severe hypoglycemia were reported.
Those data were recently published in Journal of the American Medical Association and also presented by Richard M Bergenstal, MD, executive director of the International Diabetes Center, Minneapolis, Minnesota, at the European Association for the Study of Diabetes (EASD) 2016 Annual Meeting.
"After 30-plus years of promising patients that we would have an artificial pancreas, I was never quite certain I'd see it in my career. [Now] I think we're actually going to see it in the near future," Dr Bergenstal told Medscape Medical News at the EASD conference.
However, an investigator for a competing product, Steven J Russell, MD, of the Diabetes Research Center at Massachusetts General Hospital, Boston, expressed the view that although it does reduce glycemic variability, the 670G represents a "conservative strategy" because of the ongoing need for patients to estimate their carb consumption, which can introduce error in insulin dosing.
In a statement, the FDA noted that risks associated with use of the system may include hypoglycemia and hyperglycemia, as well as skin irritation or redness around the device's infusion patch. In addition, this version of this device is unsafe for use in children 6 years of age or younger and in patients who require less than eight units of insulin per day.
As part of this approval, the FDA is requiring a postmarket study to better understand how the device performs in real-world settings. Medtronic is currently performing clinical studies to evaluate the safety and effectiveness of the device in children 7 to 13 years old with type 1 diabetes.
Dr Bergenstal has conducted research, served on a scientific advisory board, or consulted for Medtronic, Dexcom, Roche, Abbott, Johnson & Johnson, Eli Lilly, Novo Nordisk, and Sanofi. He receives no personal income from these activities, as all contracts are with his nonprofit employer HealthPartners Institute. He has inherited stock in Merck. Dr Russell has received honoraria from Tandem and Novo Nordisk, lecture and other fees from Tandem, Sanofi, Dexcom, and Eli Lilly and nonfinancial support from Dexcom, Tandem Diabetes, SweetSpot Diabetes, International Biomedical, Abbott Diabetes Care, Insulet, and Medtronic. He owns stocks/shares in Companion Medical, and has patents pending on the bionic pancreas.


